Preparing For The Coming Covid-19 And Influenza Winter Season
Monday, October 03, 2022
by John D. Tamerius, Ph.D. and Jhobe Steadman, Ph.D.
The Secretary of Health & Human Services (HHS) declared a Public Health Emergency on Jan. 31, 2020. Congress followed a few weeks later on March 13, 2020 with the declaration of a National Emergency and granted $25 billion to HHS to take immediate steps to accelerate the development of vaccines, therapeutics and diagnostic tests. Five weeks later the National Institutes of Health (NIH) created the Rapid Acceleration of Diagnostics Initiative (RADx) and Quidel® Corporation was one of several companies that received scientific and regulatory guidance and funding to help meet the profound need of the American people during this emergency. Despite the criticisms commonly heard, a tremendous amount was accomplished in record time, including the creation of effective vaccines, the development of diagnostic antigen and molecular tests for diagnosis of SARS-CoV-2 and for the advancing development of therapeutic agents---all in less than one year.
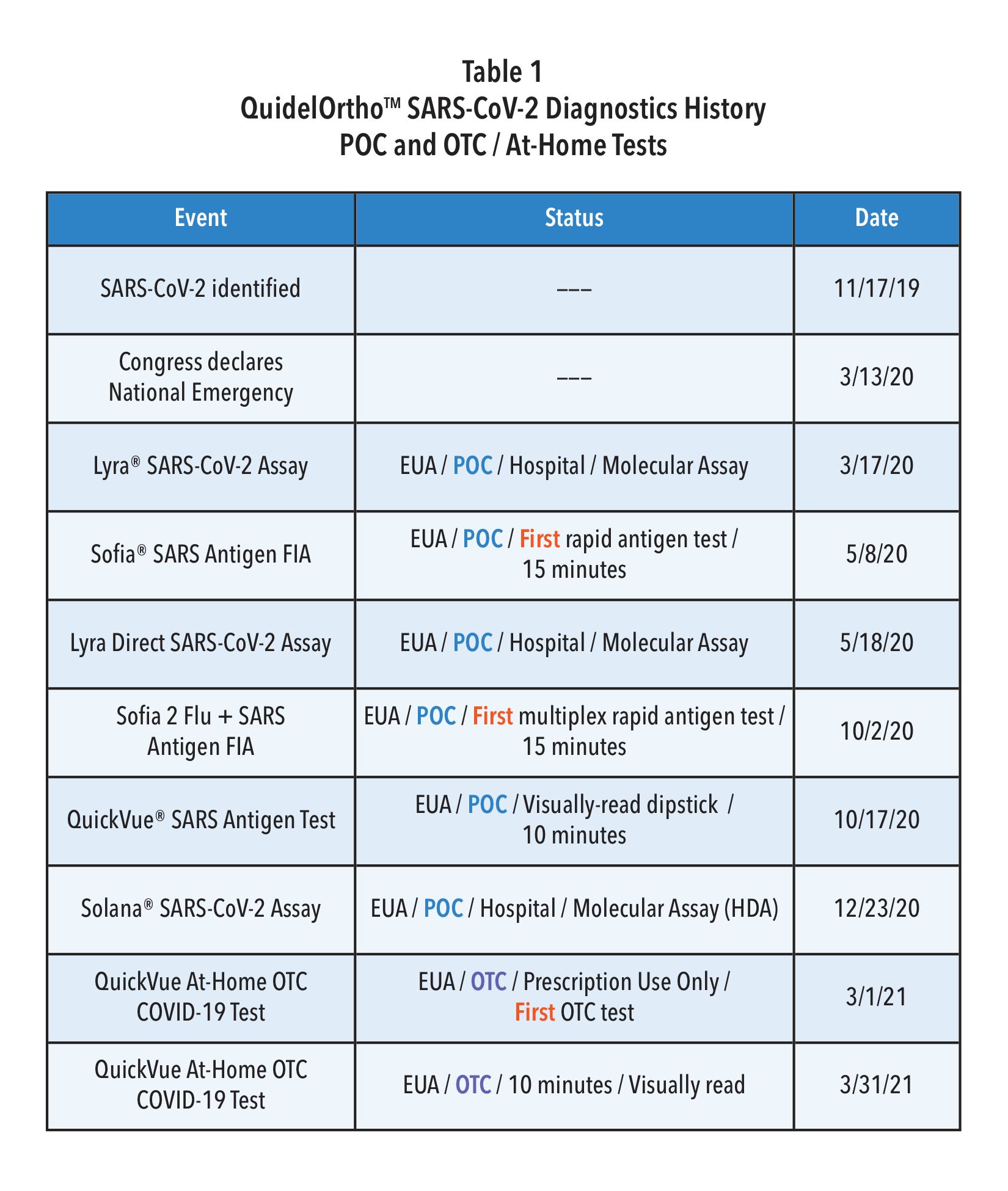
Quidel, partly with RADx support, was the first diagnostic firm to receive the FDA’s Emergency Use Authorization (EUA) for a rapid antigen test in the U.S.—the fluorescence immunoassay (FIA) called Sofia® SARS Antigen FIA for point-of-care (POC) use in the physician’s office lab. A few months later, Quidel was also the first to receive an EUA for a multiplex antigen assay, Sofia 2® Flu + SARS Antigen FIA, for simultaneous testing for SARS-CoV-2, Influenza A, and influenza B from one swab specimen. As shown in Table 1, within one year after the declaration of our National Emergency, Quidel had received EUAs for 8 different assays, including EUAs for the QuickVue SARS Assay (a 10-minute, visually read dipstick assay), one for POC and one for at-home use and two molecular assays.
This unprecedented work by government and industry required time. The pandemic was spreading quickly around the world and the public had very little to no protection. Fortunately, non-pharmaceutical interventions (NPI) were quickly introduced and promoted widely across the United States. Wherever lock downs, school closures, reduced face-to-face interaction, mask wearing, reduced mobility, and working at home occurred, there were significantly reduced hospitalizations and deaths. This protection by NPI, although not absolute, bought research scientists and industry time while vaccines, COVID-19 therapeutics and diagnostics were being developed.
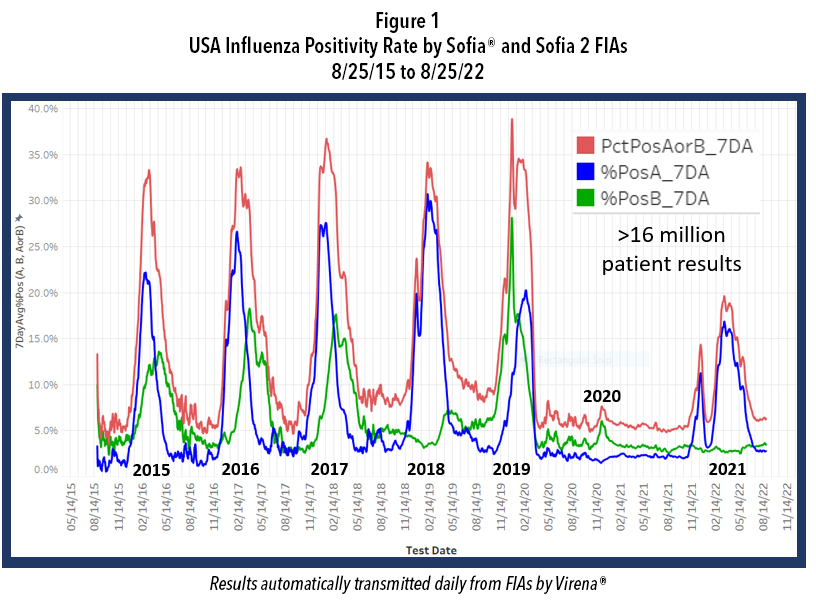
While our government agencies and industries were working on these goals, NPI also reduced the incidence of certain other respiratory infections, including that of influenza Types A and B. Figure 1 shows the course of influenza in the United States from September 2015 to the present and reveals the profound impact of NPI on influenza incidence. Note the data depicted in this figure are derived from over 16 million patient de-identified test results that were automatically transmitted to Quidel by users of Sofia and
Sofia 2 for surveillance, using a system called Virena®. Virena information is updated daily and is automatically and securely shared with the CDC and readily available to Quidel customers on MyVirena.com. Virena is also a no charge service provided to all Sofia and Sofia 2 users.
After the abrupt end of the 2019-2020 season, there followed 18 months during which the combined influenza positive rates for influenza types A and B rarely exceeded 6%. Then last winter, 2021-2022, there were two influenza surges; both failed to approach the positivity rates and the duration seen in the previous 5 epidemic seasons. Both surges this past winter also ended abruptly, timed closely to the arrivals of Omicron in Dec. 2021 and of the BA.4 and BA.5 Omicron subvariants that became dominant after January 2022. This dramatic decline in influenza is believed largely due to the implementation of NPI that were needed to reduce the spread of COVID-19 until other measures could be taken.
A large portion of our population is now feeling exhausted after doing their best to address this pandemic for over two and a half years. NPI use is on the decline. Omicron and its direct subvariant offspring are much less virulent than their predecessors, further reducing the concern about being infected, thus further reducing interest or willingness to be vaccinated or to use NPI. In addition, much of the testing for COVID-19 is now done at home and most at-home results are not reported, reducing the awareness and concern of COVID-19 in their communities. On top of this, promotional activities for NPI have diminished.
In addition to enabling COVID-19 infections to more easily spread and persist in our population, failing to use NPI will likely increase the risk of a stronger influenza season this coming winter, perhaps even resulting in a more traditional season with epidemic levels of influenza rather than the “failed” surges* we experienced last winter. Enhancing this risk, only 52% of the U.S. population is vaccinated against influenza. Even many of those who have gotten routine vaccinations for influenza annually, have possibly skipped the last year or two given the widely known low influenza incidence and, with the passing of time, whatever immunity they might have had before the advent of the COVID-19 pandemic has likely wanned. So, the risks for significant influenza season seem higher than at any time since the winter of 2019.
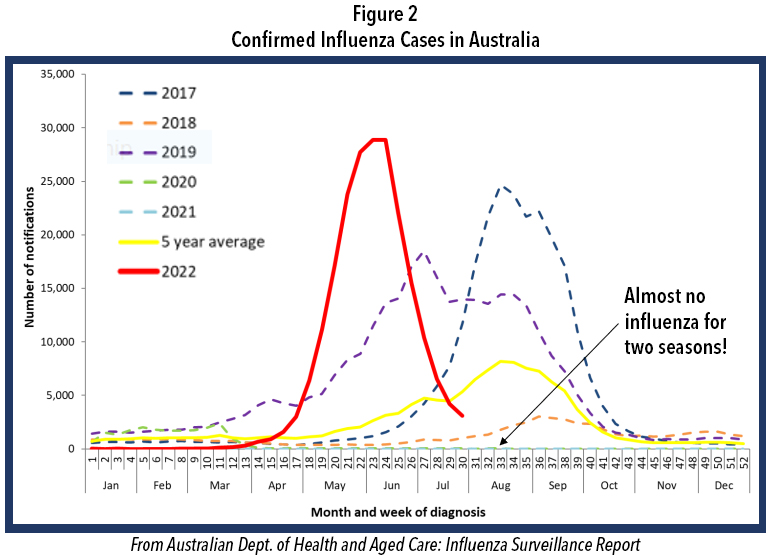
We often look to the southern hemisphere for clues because their winter precede ours by half a year. Careful examination of Figure 2 shows the profound decline in incidence of influenza in their two previous winters, just like in the USA. This winter there was a pronounced influenza epidemic and the worst influenza in May in Australia’s history. Such events in the southern hemisphere are not absolute predictors of events in the northern hemisphere, but they rightfully raise further concern for this winter in North America.
Many epidemiologists agree that a combination of both a new COVID-19 surge and a true influenza epidemic winter season in the U.S. is possible, if not likely. Indeed, it appears that the annual influenza epidemic almost broke out last 2021-2022 winter with the two surges as we discussed above. The use of NPI was likely strong enough to possibly slow or halt its spread. This may not be the case this year given the exhaustion of our populace, the low vaccination rate, reopening of schools, beginning of return to workplaces, ongoing decline in NPI use, and overall diminution of herd immunity.
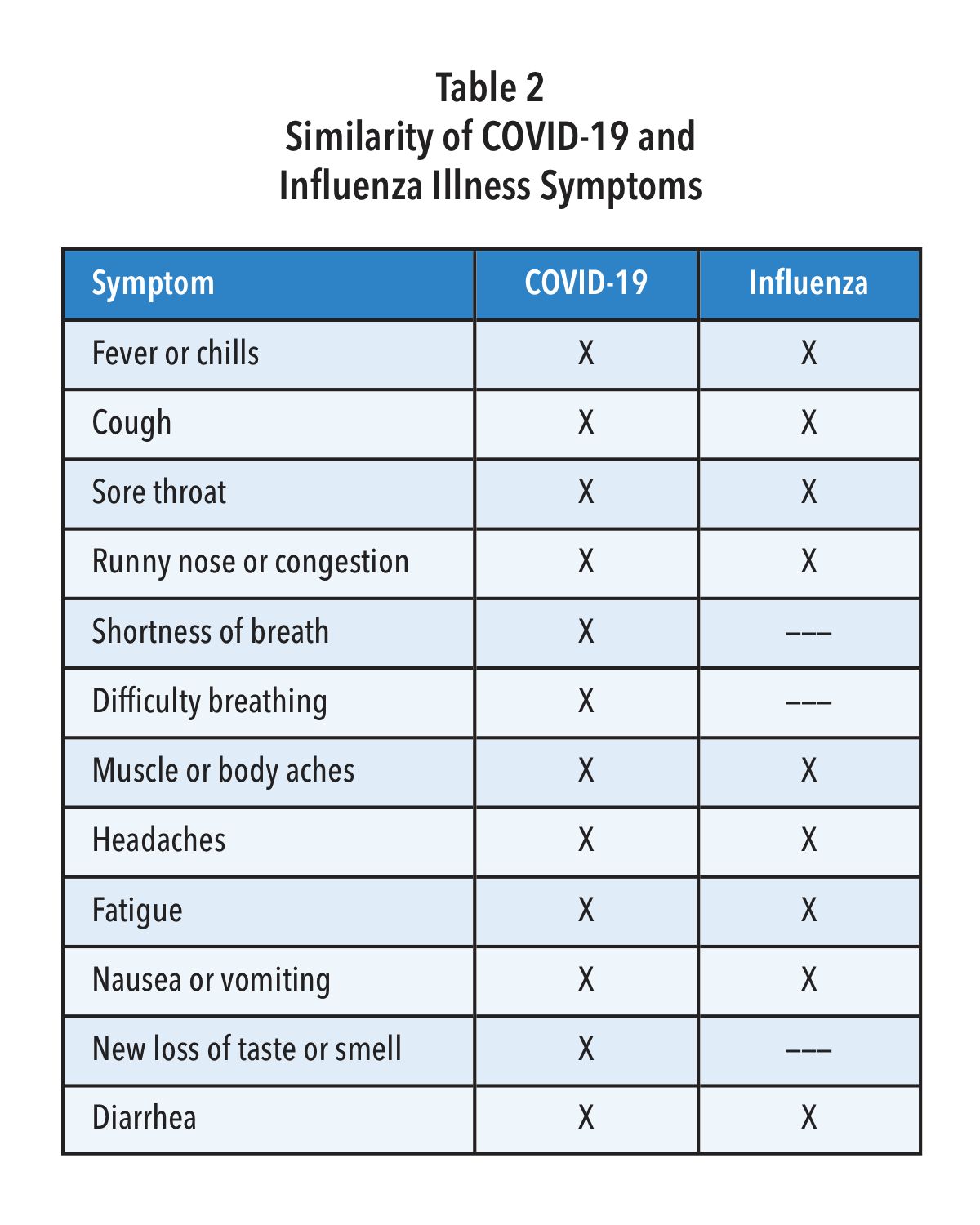
Unfortunately, the signs and symptoms for infections by SARS-CoV-2 and influenza are very similar (Table 2). Given that there are now effective treatments for both influenza and COVID-19, it seems imperative that steps are taken to test for all three pathogens when patients present with such symptoms—especially in communities where both COVID-19 and Influenza are likely circulating at the same time.
As mentioned in Table 1, Quidel was the first company to receive an EUA for a multiplex antigen detection assay in the United States. This CLIA waived test provides a result in 15 minutes with a simple nasal swab and is available for most labs. For busy clinics it provides flexible workflows, requiring limited hands-on-time, including a batch testing mode that allows processing and testing of several patient samples at a time. Getting daily reports from your local public health office or by accessing Quidel’s on-line reporting system (MyVirena.com) could indicate whether only COVID-19 or Influenza are in your community. If this is the case, options in Table 1 provide you practical and available alternatives available from Quidel.
With decline in use of NPI, reduced herd immunity, only 52% influenza vaccination rate (persons > 6 months of age), and given the hint from Australia (Figure 2), as well as the two attempts by influenza to break out in the United States more broadly last December and again in February (Figure 1), it is best to begin to prepare now for the return of influenza and potentially at the same time when COVID-19 is surging or otherwise maintaining a moderate incidence level. Rapid diagnosis is critically important as patients, especially high-risk ones, can be treated successfully with specific antivirals that have been developed for each of the pathogens—provided they are administered early, preferably starting within 2 days after symptom onset for influenza and within 5 days after symptom onset for COVID-19.
To prepare for the likely resurgence of influenza, perhaps at the same time as a new surge of COVID-19, we encourage physicians to do the following:
- Monitor surveillance data for incidence of COVID-19 and influenza in your state, county and community. This could be accomplished by monitoring public health reports and/or, for users of Sofia and Sofia 2, by monitoring the status of both viruses’ incidence by readily accessing Virena data on Quidel’s MyVirena.com.
- Make sure that your clinic provides COVID-19 and influenza test results to your local and regional public health agencies, so that they can provide a more complete status update for you and others.
- Actively support vaccinations, including boosters with the newly approved vaccine(s) covering the new SARS-CoV-2 Omicron subvariants.
- Encourage your staff and patients to get the new season’s influenza vaccines that are now available. Patients older than 65 years, should get the special influenza vaccine designed for them. Given that the influenza season started so early in Australia, it could do the same here, so providing the flu vaccinations for yourself, staff, and your patients by the end of September could be best.
- Encourage the use of NPI, especially at least through this winter, and especially reinforced by your knowledge through surveillance of incidence of both pathogens circulating in your community. The data show without question that NPI reduces COVID-10 and Influenza infections and spread.
- Be prepared for testing for both SARS-CoV-2 and Influenza. All three viruses are likely to be circulating in your community this winter—potentially at the same time. Using Sofia 2 SARS + Flu FIA may be the best choice for you, as it screens for all three viruses at once with only one patient nasal swab sample required.
*Failed “surges”. Even though the surges this past winter did not constitute a typical influenza epidemic, the CDC has reported that between Oct. 2021, and June 11, 2022, there were 8 million to 13 million influenza cases, 82,000 to 170,000 hospitalizations, and 5,000 to 14,000 deaths In the United States. We must all take steps now to prepare for the coming COVID-19 and Influenza winter season.
